Redox state of the solid Earth
We’re interested in constraining the redox state of the solid Earth using the behavior of multivalent elements as proxies for oxygen fugacity. Oxygen fugacity is a thermodynamic parameter that controls, to a first order, the structure of the planet and the chemistry of rocks, ores and the atmosphere. This research often takes us to the synchrotron (Advanced Photon Source) at Argonne National Lab to characterize our samples.
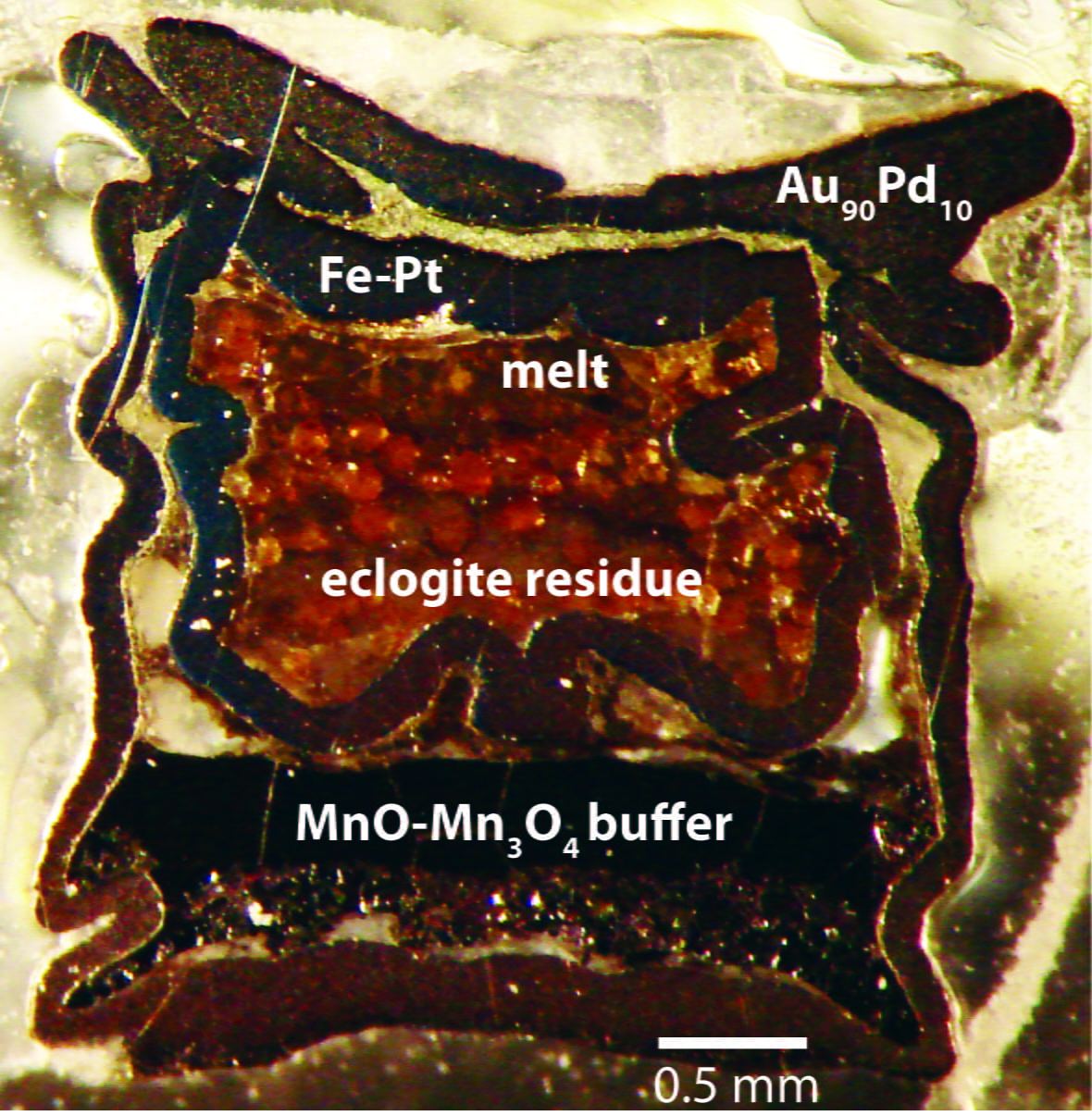
This image shows a thin section cross section of a high pressure oxygen-buffered experiment utilizing a double-capsule technique. The interior FePt capsule contains our sample of interest: a synthetic eclogite (high pressure metamorphic rock) surrounded by hydrous silicate melt.
Diffusion geospeedometry
Equilibrium changes in pressure, temperature and composition redistribute cations and their isotopes in the solid Earth. However, the extent of chemical exchange in response to shifting conditions will be limited by diffusion, which controls the transportation of elements in geologic materials over the nanometer-to-meter scale. We calibrate diffusion coefficients in the lab to understand how kinetic phenomena preserved in natural samples record the tempos of heating, alteration and exhumation events in magmatic and metamorphic systems.
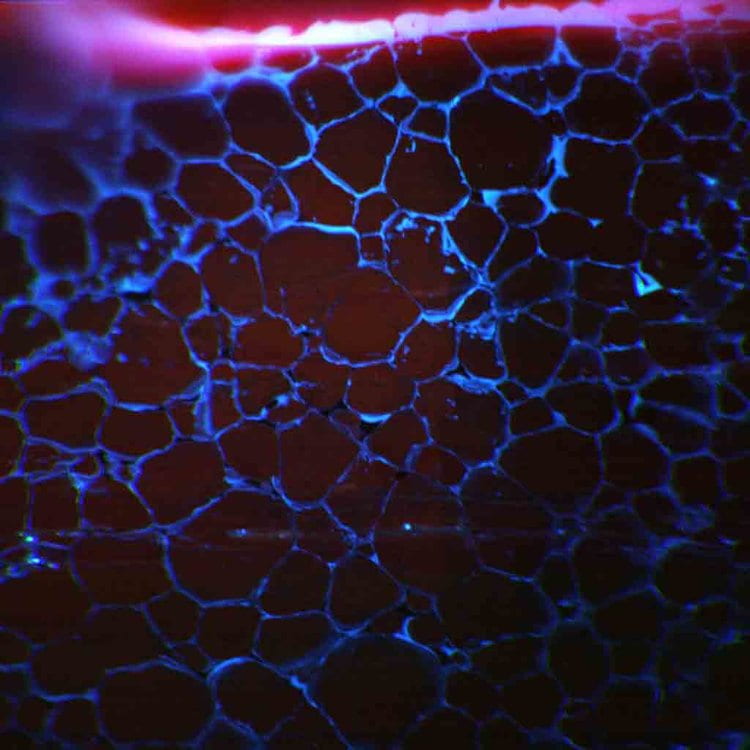
Shown to the left is an RGB cathodoluminescence (CL) image of an experiment measuring the diffusivity of a Ti in quartz grain boundaries. The blue color is from the presence of titanium, a CL activator, in the region adjacent to the crystalline grain boundaries.